Building Blocks of Life's Building Blocks Come From Starlight
Feature • October 12, 2016
Life exists in a myriad of wondrous forms, but if you break any organism down to its most basic parts, it's all the same stuff: carbon atoms connected to hydrogen, oxygen, nitrogen and other elements. But how these fundamental substances are created in space has been a longstanding mystery.
Now, astronomers better understand how molecules form that are necessary for building other chemicals essential for life. Thanks to data from the European Space Agency's Herschel Space Observatory, scientists have found that ultraviolet light from stars plays a key role in creating these molecules, rather than "shock" events that create turbulence, as was previously thought.
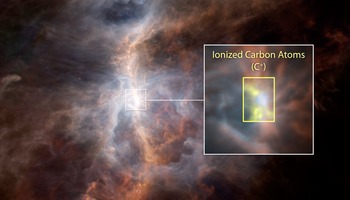
Scientists studied the ingredients of carbon chemistry in the Orion Nebula, the closest star-forming region to Earth that forms massive stars. They mapped the amount, temperature and motions of the carbon-hydrogen molecule (CH, or “methylidyne” to chemists), the carbon-hydrogen positive ion (CH+) and their parent: the carbon ion (C+). An ion is an atom or molecule with an imbalance of protons and electrons, resulting in a net charge.
"On Earth, the sun is the driving source of almost all the life on Earth. Now, we have learned that starlight drives the formation of chemicals that are precursors to chemicals that we need to make life," said Patrick Morris, first author of the paper and researcher at the Infrared Processing and Analysis Center at Caltech in Pasadena.
In the early 1940s, CH and CH+ were two of the first three molecules ever discovered in interstellar space. In examining molecular clouds -- assemblies of gas and dust -- in Orion with Herschel, scientists were surprised to find that CH+ is emitting rather than absorbing light, meaning it is warmer than the background gas. The CH+ molecule needs a lot of energy to form and is extremely reactive, so it gets destroyed when it interacts with the background hydrogen in the cloud. Its warm temperature and high abundance are therefore quite mysterious.
Why, then, is there so much CH+ in molecular clouds such as the Orion Nebula? Many studies have tried to answer this question before, but their observations were limited because few background stars were available for studying. Herschel probes an area of the electromagnetic spectrum -- the far infrared, associated with cold objects -- that no other space telescope has reached before, so it could take into account the entire Orion Nebula instead of individual stars within. The instrument they used to obtain their data, HIFI, is also extremely sensitive to the motion of the gas clouds.
One of the leading theories about the origins of basic hydrocarbons has been that they formed in "shocks," events that create a lot of turbulence, such as exploding supernovae or young stars spitting out material. Areas of molecular clouds that have a lot of turbulence generally create shocks. Like a large wave hitting a boat, shock waves cause vibrations in material they encounter. Those vibrations can knock electrons off atoms, making them ions, which are more likely to combine. But the new study found no correlation between these shocks and CH+ in the Orion Nebula.
Herschel data show that these CH+ molecules were more likely created by the ultraviolet emission of very young stars in the Orion Nebula, which, compared to the sun, are hotter, far more massive and emit much more ultraviolet light. When a molecule absorbs a photon of light, it becomes "excited" and has more energy to react with other particles. In the case of a hydrogen molecule, the hydrogen molecule vibrates, rotates faster or both when hit by an ultraviolet photon.
It has long been known that the Orion Nebula has a lot of hydrogen gas. When ultraviolet light from large stars heats up the surrounding hydrogen molecules, this creates prime conditions for forming hydrocarbons. As the interstellar hydrogen gets warmer, carbon ions that originally formed in stars begin to react with the molecular hydrogen, creating CH+. Eventually the CH+ captures an electron to form the neutral CH molecule.
"This is the initiation of the whole carbon chemistry," said John Pearson, researcher at NASA's Jet Propulsion Laboratory, Pasadena, California, and study co-author. "If you want to form anything more complicated, it goes through that pathway."
Scientists combined Herschel data with models of molecular formation and found that ultraviolet light is the best explanation for how hydrocarbons form in the Orion Nebula.
The findings have implications for the formation of basic hydrocarbons in other galaxies as well. It is known that other galaxies have shocks, but dense regions in which ultraviolet light dominates heating and chemistry may play the key role in creating fundamental hydrocarbon molecules there, too.
"It's still a mystery how certain molecules get excited in the cores of galaxies," Pearson said. "Our study is a clue that ultraviolet light from massive stars could be driving the excitation of molecules there, too."
Herschel is a European Space Agency mission, with science instruments provided by consortia of European institutes and with important participation by NASA. While the observatory stopped making science observations in April 2013, after running out of liquid coolant as expected, scientists continue to analyze its data. NASA's Herschel Project Office is based at NASA’s Jet Propulsion Laboratory, Pasadena, California. JPL contributed mission-enabling technology for two of Herschel's three science instruments. The NASA Herschel Science Center, part of IPAC, supports the U.S. astronomical community. Caltech manages JPL for NASA.